
One dangerous aspect of corrosion is that it can compromise the stability of structures, which is particularly relevant in the naval industry, where material failure leads to leaks. However, another danger of corrosion has recently become apparent.
The Challenges of Using Cathodic Protection on Submarines
Galvanic, crevice, and other types of corrosion are electrochemical in nature and give rise to small currents passing between the corroding and most noble regions via the material and surrounding electrolyte. To combat this, you can impose the opposite electric current in a process known as cathodic protection. In many cases, this leads to an even larger current passing through the material and surrounding electrolyte.
This is where problems arise. Electric currents give rise to electric potentials, which can be measured. If a submarine, for example, is imposing an electric current through its hull and propeller, then there is a pretty big (in terms of area) electric potential that could be used to detect the submarine, or even set off a mine. This is further complicated by the submarine’s rotating propeller, which modulates the signal.
What can we do to address this issue? One solution is to disable cathodic protection when the submarine is in an area of reasonable danger. However, this won’t work because the actual corrosion process itself has its own electric potential signature that can be detected.
Simulating the Electrical Potential Signatures of Submarines
David Schaefer at the University of Duisburg-Essen, Germany, simulates the underwater electrical potential signatures of submarines on the commission of the Technical Center for Ships and Naval Weapons. Using the COMSOL Multiphysics® software, he and his team were able to simulate the signal and determine that cathodic protection could be run at a certain amperage, resulting in a signal that is less noticeable than that during corrosion. Read more about the research in the COMSOL News 2012 article “Submarines: Corrosion Protection or Enemy Detection?” starting on page 67.
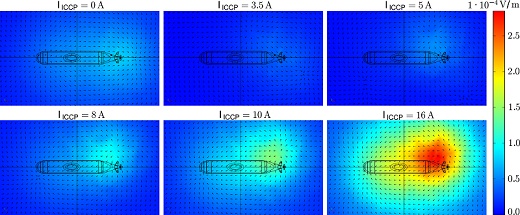
Underwater electric potential signatures below the keel for different currents imposed through cathodic protection. A signature is evident when corrosion is occurring (top left), which can be optimized at 3.5 A (top middle). Overprotection results in larger signatures.
Further Reading
Learn more about different applications of corrosion modeling on the COMSOL Blog:



Comments (1)
swapnil more
February 16, 2021Have you any idea about Volatile Chemical Inhibitors – https://www.safepack.com/volatile-corrosion-inhibitor/