Copper Electroless Deposition
Application ID: 19863
Electroless deposition or plating is a non-galvanic plating method that does not require any external electrical power. This technique is typically used for electroless plating of nickel, silver, gold and copper.
In electroless deposition, partial oxidation and reduction reactions occur at the same electrode surface. The potential difference that exists between the equilibrium potentials for oxidation and reduction reactions and the potential at the electrode surface is the driving force for deposition process.
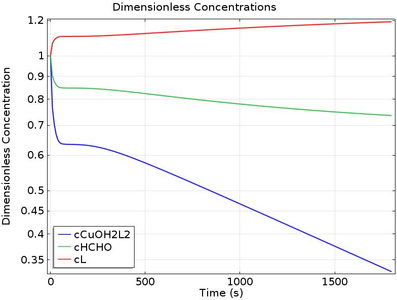
This tutorial predicts the change in current density, deposition thickness and concentration of ionic species during electroless deposition.
この model の例は, 通常次の製品を使用して構築されるこのタイプのアプリケーションを示しています.
ただし, これを完全に定義およびモデル化するには, 追加の製品が必要になる場合があります. さらに, この例は, 次の製品の組み合わせのコンポーネントを使用して定義およびモデル化することもできます.
- COMSOL Multiphysics® and
- either the バッテリデザインモジュール, 腐食解析モジュール, 電気化学モジュール, 電気めっきモジュール, or 燃料電池&電解槽モジュール
アプリケーションのモデリングに必要な COMSOL® 製品の組み合わせは, 境界条件, 材料特性, フィジックスインターフェース, パーツライブラリなど, いくつかの要因によって異なります. 特定の機能が複数の製品に共通している場合もあります. お客様のモデリングニーズに適した製品の組み合わせを決定するために, 製品仕様一覧 を確認し, 無償のトライアルライセンスをご利用ください. COMSOL セールスおよびサポートチームでは, この件に関するご質問にお答えしています.
