燃料電池 & 電解槽モジュールアップデート
燃料電池 & 電解槽モジュールのユーザー向けに, COMSOL Multiphysics® バージョン 6.4 では, 新しいパワー損失変数, 電極反応での化学種の消費に比例したガス流入を定義する機能, およびより柔軟な化学種モデリング手法が導入されました. これらのアップデートやその他の詳細については, 以下をご覧ください.
流入口での化学量論フィード
動作中の燃料電池または電解槽における一般的な状況, すなわち, ガス混合物の入口流量がセル電流に比例して設定され, 過剰な化学種が確実に消費される状況をモデル化するために, 水素燃料電池 および 水電解槽 インターフェースの H2 流入 および O2流入 ノードに 化学量論フィード チェックボックスが追加されました. この追加機能は, 以下のチュートリアルモデルで確認できます:
O2-in-H2 および H2-in-O2 混合
劣化, 寄生反応, および起動・停止シナリオに関連するより高度なモデルを可能にするために, 水素燃料電池 および 水電解槽 インターフェースでは, O2 混合気体 設定で H2 をガス種として有効にし, H2 混合気体 設定で O2 をガス種として有効にできるようになりました. このアップデートは, 高分子電解質膜燃料電池における炭素腐食 チュートリアルモデルで確認できます.
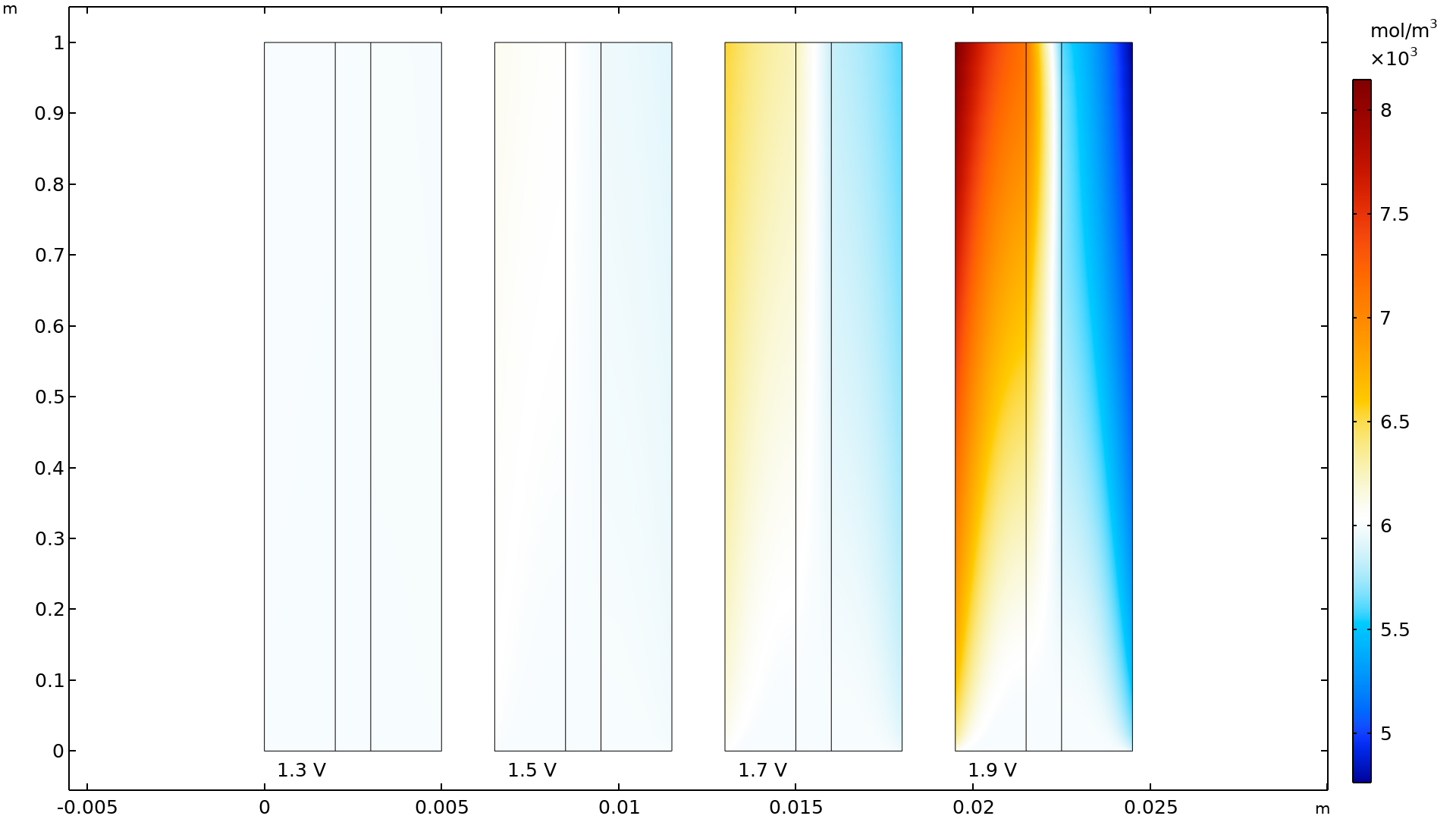
電力損失評価変数
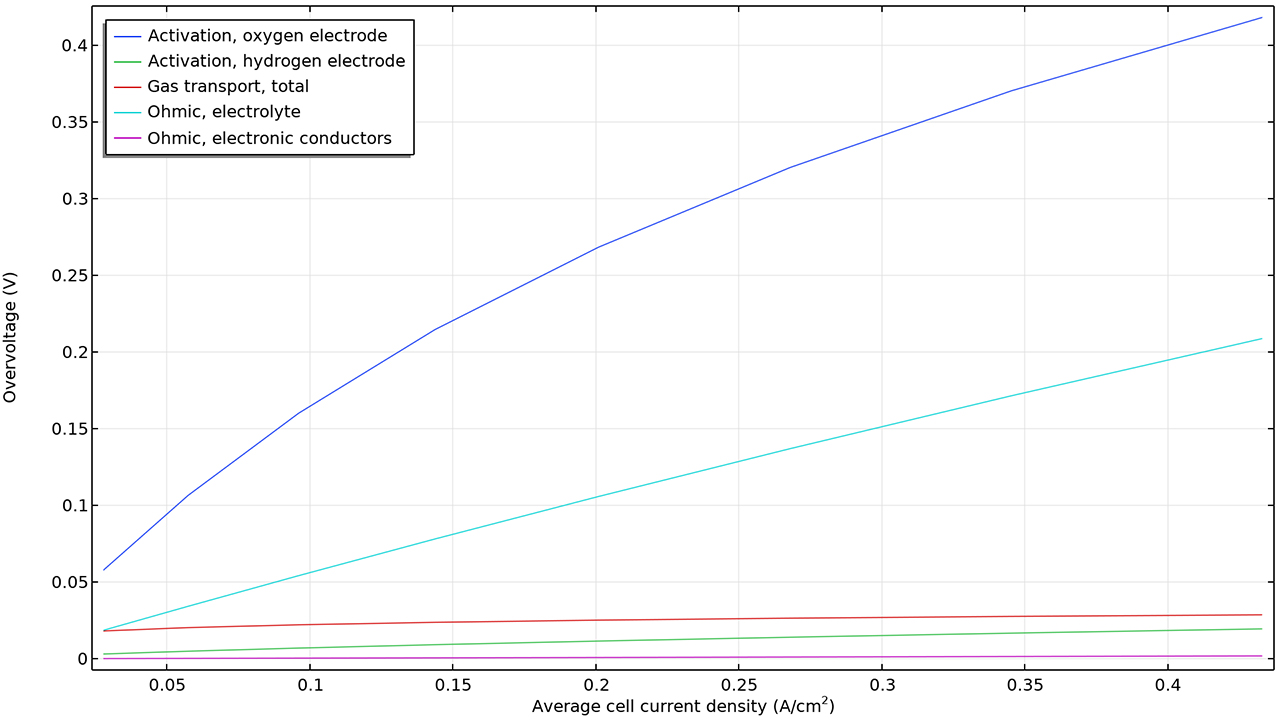
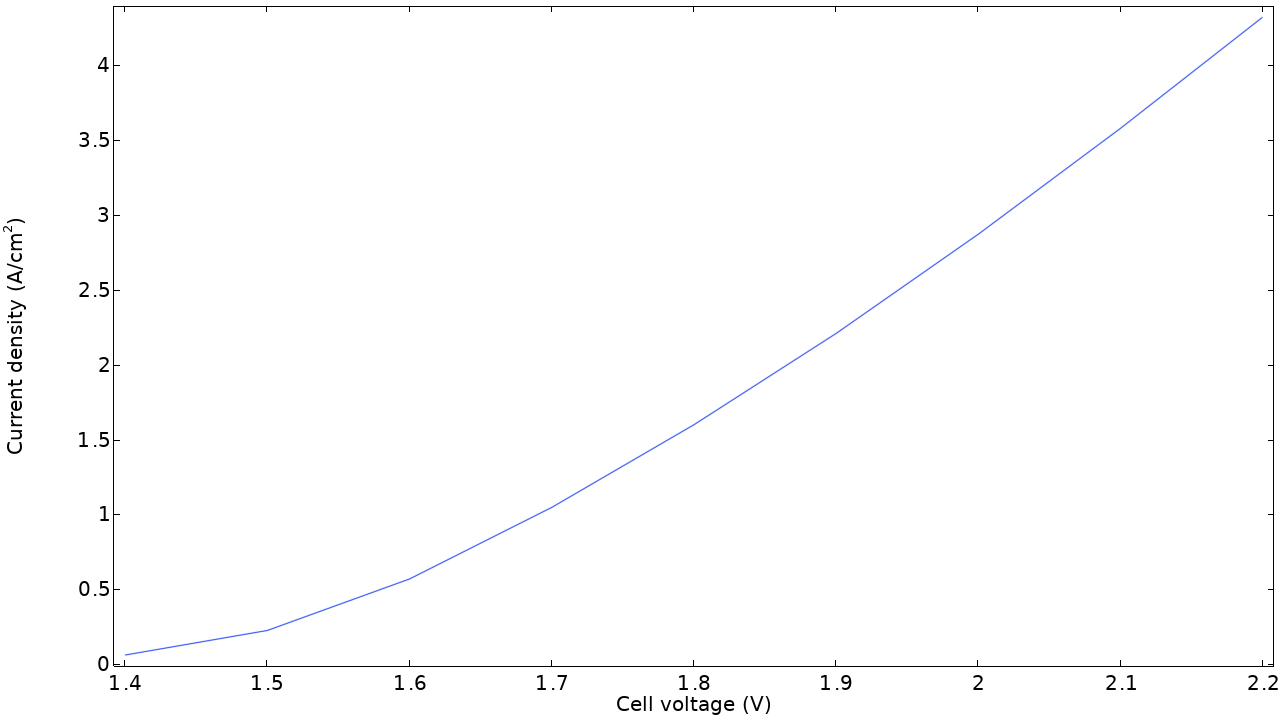
電気化学 インターフェース類に新しく導入された電力損失変数を使用して, 燃料電池または電解槽における総電力損失の大きさを評価し, 電解質, 電極, 電流導体などの個々のコンポーネント間の損失を比較できるようになりました.
電力損失は, すべての反応種および輸送種のギブズ自由エネルギーの損失を考慮することによって定義され, 抵抗性損失, 濃度損失, および活性化損失を区別することが可能です. これらの変数は, ドメインや境界上でのローカルな値, セル全体での積分値, もしくは個々のモデルツリー機能ノードごとの値として利用可能です.
この機能は, チュートリアルモデル 燃料電池カソードにおける質量輸送と電気化学反応 および 高分子電解質膜燃料電池における炭素腐食 で確認できます.
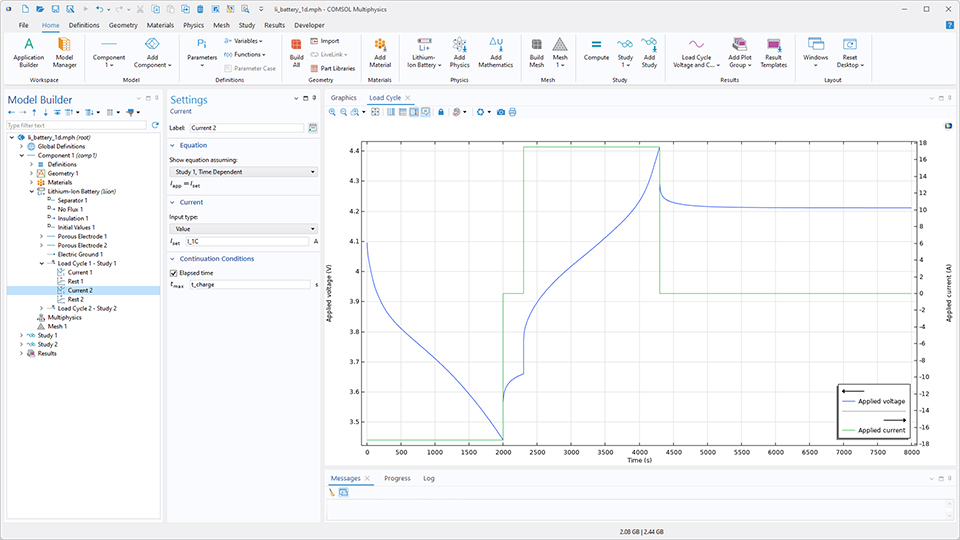
負荷サイクル
複雑なサイクル設定の簡便なセットアップを可能にするため, ほとんどの 電気化学 インターフェースに新たな負荷サイクル機能が追加されました. この機能を使用すると, 電圧, パワー, 電流, Cレート, 休止 ステップを任意の順序で追加し, 任意の充放電負荷サイクルを定義できます. 負荷サイクルの各ステップでは, 時間, 電圧, 電流の制限値, および任意の変数式を用いたユーザー定義条件に基づく, 1つまたは複数の動的継続/中断 (切り替え) 基準を定義できます. 多様な負荷サイクル定義オプションに加え, この新機能では電流・電圧プローブの自動定義やソルバー停止条件の設定も可能です.
サブループ サブ機能を使用すると, 例えば, 長期の充放電サイクル試験と基準性能試験を組み合わせて実施することができます. なお, パワー および サブループ サブ機能は, バッテリデザインモジュールおよび燃料電池 & 電解槽モジュールでのみご利用いただけます.
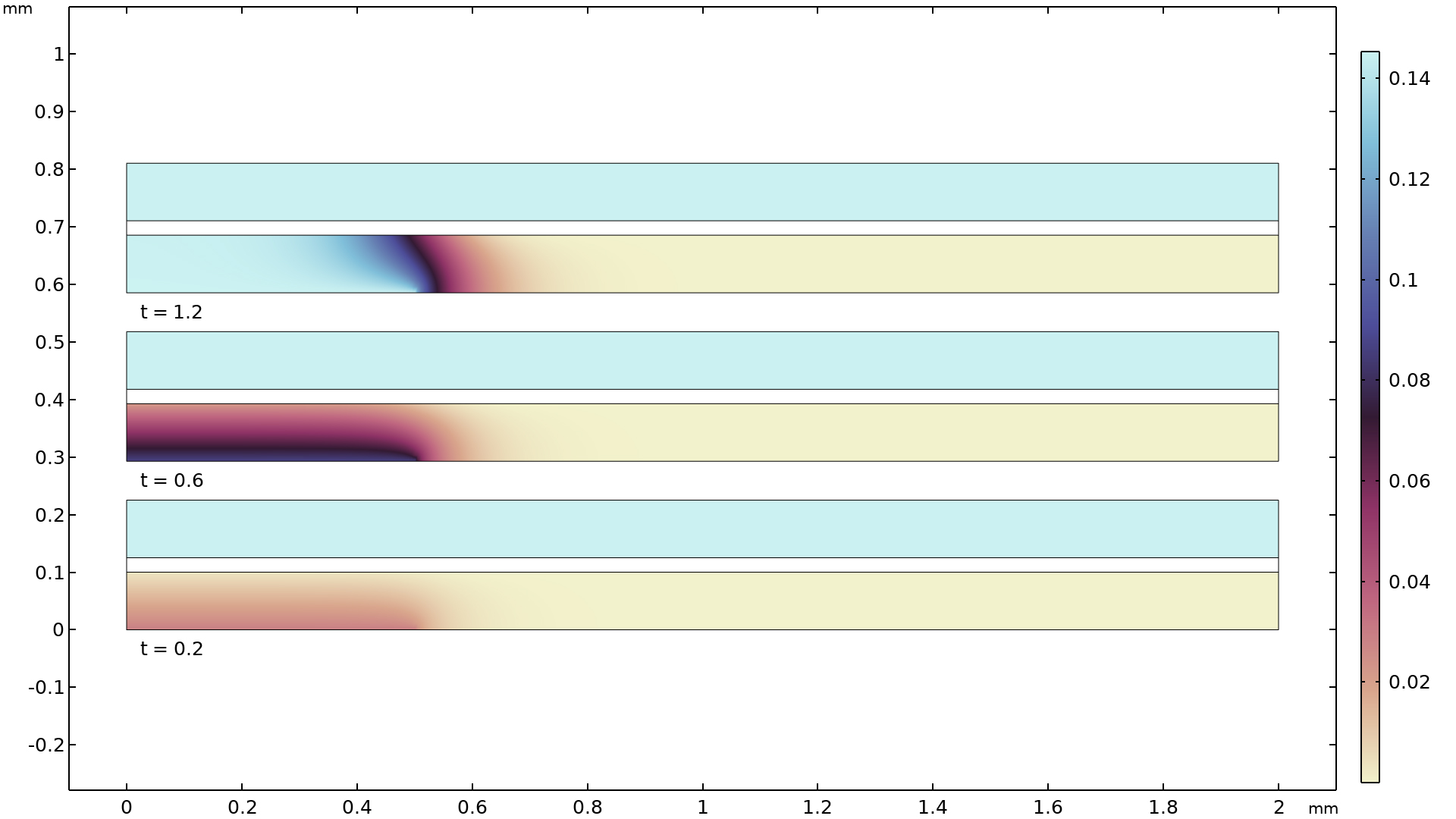
水性電解質輸送
弱酸, 弱塩基, 両性イオン, および一般的な複合種を特徴とする水性電解質のモデリング, ならびに機構論的腐食モデリング, 生物系の電気化学モデル, 電気化学センサーモデリングなどの用途向けに, 新しい 水性電解質輸送 インターフェースが追加されました. これは, 希薄水性電解質中の電位および化学種の濃度場を計算します. この輸送は, 拡散, マイグレーション, 対流に加え, 電気中性と水の自己イオン化平衡反応 (自己プロトロシス) を組み込んだネルンスト・プランク方程式によって定義されます. 方程式反応のより効率的な処理とモデル設定の容易さから, この新しいインターフェースは, より汎用的な 3次電流分布 (ネルンスト・プランク) インターフェースよりも, 使用が適当な場合があります.
イオン交換膜モデルの自動初期化
電気的中性とドナン平衡の遵守のため, 3次電流分布 (ネルンスト・プランク) インターフェースの イオン交換膜 機能に 初期値に Donnan シフトを追加 オプションが追加されました. このオプションは, 有効な イオン交換膜 ドメインノードの 初期値 機能で指定された初期濃度および電位値を自動的にシフトします. これは, ユーザー定義値が膜と平衡状態にあるバルク液体電解質の値を表すと仮定しています. シフトされた初期値はソルバーの初期値として使用されます. このオプションを有効にすると, 追加の解析ステップで膜の固定空間電荷を所定の非ゼロ値までスイープする必要がなくなるため, モデル設定が簡略化されます. この機能は 電気透析セルでの脱塩 チュートリアルモデルでご覧いただけます.
周期条件
ダルシー則 および リチャーズ方程式 インターフェースに新たな 周期条件 機能が追加され, 2つ以上の境界間の流れに対して周期性を容易に強制できるようになりました. さらに, 圧力ジャンプを直接指定するか, 質量流量を規定することで, ソース境界と行先境界間に圧力差を生成することが可能です. 周期条件は, 代表体積要素のモデル化や, 均質化された多孔質媒体の有効特性の計算に一般的に用いられます.
自由および多孔質流れカップリングにおける圧力ジャンプオプション
自由および多孔質流れ カップリングには, 自由媒体と多孔質媒体の境界における圧力ジャンプを考慮する新オプションが追加されました. これにより, 例えば多孔質スペーサー材料で支持された半透膜における浸透圧や, 多相流における毛管圧力による圧力ジャンプなどのモデル化が可能になります.
新規および更新されたチュートリアルモデル
COMSOL Multiphysics® バージョン 6.4 では, 燃料電池 & 電解槽モジュールのチュートリアルモデルが新規追加および更新されました.